A novel role for nuclear caspase-8 in tumor progression
Until recently, metastatic melanoma (MM) was considered refractory to treatment with a 3-year survival below 10%. A better understanding of the genetic alterations in MM cells has fundamentally changed the systemic therapy and significantly improved the prognosis of patients. Combinations of mutBRAF inhibitors plus MEK inhibitors are currently accredited in the clinic for the treatment of mutBRAF melanoma showing a disease control rate of ̴ 95% and improved median survival. However, the vast majority of patients acquire resistance, resulting in tumor relapse. No targeted therapeutics exist to date to effectively treat patients with BRAF wildtype (wtBRAF) MM. Due to retarded onset of immune checkpoint inhibition, response rates especially of patients with wtBRAF and mutant NRAS MM (wtBRAF/mutNRAS, ~30% of patients) remain rather low, and patients present with enhanced tumor progression. Thus, targeted therapies as well as immune checkpoint inhibition currently provide good therapeutic options for the treatment of MM, offering a long-term survival to 30% of the patients. Still, 70% of patients succumb to the disease, reflecting the unmet medical need for the identification of alternative therapeutic targets.
Mutations in p53 is a hallmark of tumor development and found in ~50% of all tumors, leading to loss-of-function or gain-of-function of this important tumor suppressor. As a consequence, cancer cells resist p53-dependent cell cycle checkpoints and intrinsic apoptosis. Intriguingly, the residual 50% of tumors can still progress with wildtype p53 (wtp53) indicating that these tumor cells must be able to adapt alternative mechanisms suppressing wtp53 functions. Paradoxically, even though MM only represents about 5% of all skin tumors, it is responsible for 90% of deaths caused by skin cancer, but only 19% of MM carry 53 mutations.
Cytosoliccaspase-8 is well defined as being key driver of extrinsic apoptotic cell death. Accordingly, vitalizing pro-apoptotic pathways by reactivation or overexpression of caspases has been implemented as anti-tumor strategies. Intriguingly, more recently accumulating studies suggest that some cancer types are able to utilize alternative functions of caspases to their advantage, thereby enabling survival, proliferation and metastasis.
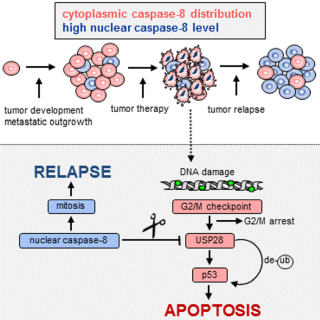
Aiming to elucidate the contribution of caspase-8 to cancer progression we were able to unravel the molecular mechanism how caspase-8 expression and sub-cellular localization may contribute to the progression of p53 proficient tumors and be causative for therapy resistance and tumor relapse. We have recently identified nuclear caspase-8 to lower the expression of wtp53 in aggressive tumor cells. We were able show that stabilization of wtp53 is facilitated by the de-ubiquitinating enzyme (DUB) USP28, and provided evidence that tumor cells expressing high nuclear levels of caspase-8 have defective p53-dependent apoptosis because caspase-8 cleaves and inactivates USP28 in cells with delayed or compromised mitosis (see figure).
Relevant own publications
- Montinaro A, Zubiaur IA, Saggau J, Kretz A-L, Ferreira RMM, Hassan O, Kitzig E, Mueller I, El-Bahrawy M, von Karstedt S, Kulms D, Liccardi G, Lemke J, Walczak H: Potent pro-apoptotic combination therapy is highly effective in a broad range of cancers. Cell Death Differ. 2022 Mar;29(3):492-503. Epub 2021 Sep 17.
doi: 10.1038/s41418-021-00869-x. (2022) - Müller I, Strozyk E, Schindler S, BeissertS, Zarni OoH, SauterT, LucarelliP, RaethS, HausserA, Al NakouziN, FazliL, GleaveM, LiuH, SimonHU, WalczakH, GreenDR, BartekJ, DaugaardM, KulmsD: Cancer cells employ nuclear caspase-8 to promote tumor progression by overcoming the p53-dependent G2/M checkpoint through cleavage of the de-ubiquitinase USP28. Mol Cell, doi: 10.1016/j.molcel.2019.12.023. (2020)
- Margue C, Philippidou D, Kozar I, Cesi G, Felten P, Kulms D, Letellier E, Haan C, Kreis S: Kinase inhibitor library screening identifies synergistic drug combinations effective in sensitive and resistant melanoma cells. J Exp Clin Cancer Res, 38:56. doi: 10.1186/s13046-019-1038-x (2019)
- RožancJ, SakellaropoulosT, AntoranzA, GuttàC, PodderB, VetmaV, PliakaV, SauterT, KulmsD, RehmM, AlexopoulosLG: Phosphoprotein patterns allow predicting of trametinib responsiveness and optimal trametinib sensitization strategies in melanoma. Cell Death Differ, doi: 10.1038/s41418-018-0210-8 (2018)
- Niessner H, Sinnberg T, Smalley KSM, Beck D, Praetorius, Mai M, Beissert S, Kulms D, Schaller M, Garbe C, Flaherty K, Westphal D, Wanke I, Meier F: BRAF inhibitors amplify the pro-apoptotic activity of MEK inhibitors by inducing ER stress in NRAS-mutant melnaoma. Clin Cancer Res, 23:6203-6214. doi: 10.1158/1078-0432.CCR-17-0098. (2017)
- Niessner H, Schmitz J, Tabatabai G, Schmid AM, Calaminus C, Sinnberg T, Weide B, Garbe C, Schittek B, Quintanilla-Fend L, Mai M, Praetorius C, Beissert S, Schackert G, Muders M, Meinhardt M, Baretton G, Dummer R, Flaherty K, Pichler BJ, Kulms D, Westphal D, Meier F: PI3K pathway inhibition achieves potent antitumor activity in melanoma brain metastases in vitro and in vivo. Clin Cancer Res, doi: 10.1158/1078-0432.CCR-16-0064. (2016)
- Niessner H, Forschner A, Klumpp B, Honegger JB, Witte M, Bornemann A, Dummer R, Adam A, Bauer J, Tabatabai G, Flaherty K, Sinnberg T, Beck D, Leiter U, Mauch C, Roesch A, Weide B, Eigentler T, Schadendorf D, Garbe C, Kulms D, Quintanilla-Martinez L, Meier F: Targeting hyperactivation of the AKT survival pathway to overcome therapy resistance of melanoma brain metastases. Cancer Med,doi: 10.1002/cam4.50. (2013)
- Beck D, Niessner H, Smalley KS, Flaherty K, Paraiso KH, Busch C, Sinnberg T, Vasseur S, Iovanna JL, Drießen S, Stork B, Wesselborg S, Schaller M, Biedermann T, Bauer J, Lasithiotakis K, Weide B, Eberle J, Schittek B, Schadendorf D, Garbe C, Kulms D, Meier F: Vemurafenib potently induces endoplasmic reticulum stress-mediated apoptosis in BRAFV600E melanoma cells. Sci Signal, 6(260):ra7. doi: 10.1126/ scisignal.2003057. (2013)
- Niessner H, Sinnberg T, Beck D, Lasithiotakis K, Maczey E, Venturelli S, Berger A, Mauthe M, Toulany M, Flaherty K, Schaller M, Schadendorf D, Proikas-Cezanne T, Schittek B, Garbe C, Kulms D, Meier F: The farnesyl transferase inhibitor lonafarnib inhibits mTOR signalling and enforces sorafenib-induced apoptosis in melanoma cells. J Invest Dermatol 131: 468-479 doi.org/10.1038/jid.2010.297. (2011)
- Sinnberg T, Lasithiotakis K, Niessner H, Schittek B, Flaherty K, Kulms D, Maczey E, Campos M, Gogel J, Garbe C, Meier F: Inhibition of PI3K-AKT-mTOR signalling sensitizes melanoma cells to cisplatin and temozolomide. J Invest Dermatol 129: 1500-1515. doi.org/10.1038/jid.2008.379. (2009)
- Lasithiotakis KG, Sinnberg TW, Schittek B, Flaherty KT, Kulms D, Maczey E, Garbe C, Meier F: Combined Inhibition of MAPK and mTOR signalling inhibits growth, induces cell death, and abrogates invasive growth of melanoma cells.J Invest Dermatol, 128: 2013-2023. doi.org/10.1038/jid.2008.44. (2008)
- Meier F, Busch S, Lasithiotakis K, Kulms D, Garbe C, Maczey E, Herlyn M, Schittek B: Combined targeting of MAPK and AKT signalling pathways is a promising strategy for melanoma treatment. Br J DermatolJun;156(6):1204-13. Epub 2007 Mar 28. 1204-1213. doi.org/10.1111/j.1365-2133.2007.07821.x (2007)


